It’s been months since researchers in China sequenced the novel coronavirus now known as SARS-CoV-2 or COVID-19. With billions of lives and trillions of dollars at stake just depending on a vaccine for COVID-19, scientists around the world are working 24×7 to create an effective and safe vaccinne as quickly as possible.
However, by some optimistic estimates, the world could see a vaccine in the not too distant future. But with every vaccine, there are unpredictable unknowns. Or worst case scenario, scientists may not be able to manufacture a vaccine at all.
Major Key Points and Success So Far:
- Eight potential COVID-19 vaccines are being tested in human clinical trials out of which 2 are being tested in the U.S., 4 are being tested in China, and 1 is being tested in the U.K. In fact, 1 is being tested in both the U.S. and Europe. (Voanews, 2019)
- One hundred and two other vaccines are being explored, but they haven’t made it to human clinical trials yet or may enter this stage soon.
- Worldwide researchers claim that we’re likely 12 to 18 months away from a vaccine but Bill Gates, whose foundation is supporting research efforts, believes it could take as little as nine months.
What is a vaccine?
A vaccine helps prevent a disease or reduce the risk of infection. It works with your body’s natural defense system to build immunity. According to the Centers for Disease Control and Prevention, a vaccine contains ingredients that imitate an infection in your body. It could be a weakened or dead virus or bacteria, or parts of a virus or bacteria. Though vaccines can cause side effects, they do not make you sick with the illness you’re vaccinating against. But they do cause your body to produce immune cells called macrophages, T-lymphocytes, and B-lymphocytes to fight off the disease. (CDC Guidelines, 2020)
After getting a vaccine, it takes your body time to build up protection. If you are exposed to the actual virus or bacteria in the future, your body remembers how to fight it.
In the current scenario, some vaccines are good for life while others may require a booster shot after some time.
How long does it take to develop a vaccine?
Developing an immunization takes hundreds of millions of dollars in investment, and very few projects result in success. Global manufacturing and distribution, meanwhile, will cost billions more. However, the economic cost of the coronavirus will be in the trillions; the cost to the U.S. alone has been estimated at $1.5 trillion in annual output. (Wired, 2020)
It usually takes about 10 to 15 years — and sometimes longer — to develop a vaccine. Why so long? Because vaccines go through a multistage research process that includes several rounds of testing. Numerous groups of people are involved in vaccine development. Health professionals, academia, manufacturers, and private industry workers all help put vaccines together. Government agencies, non-governmental organizations, and media persons also aid with the development process. Finally, individuals and communities are crucial in both development and testing.
The Phases of Vaccine Development
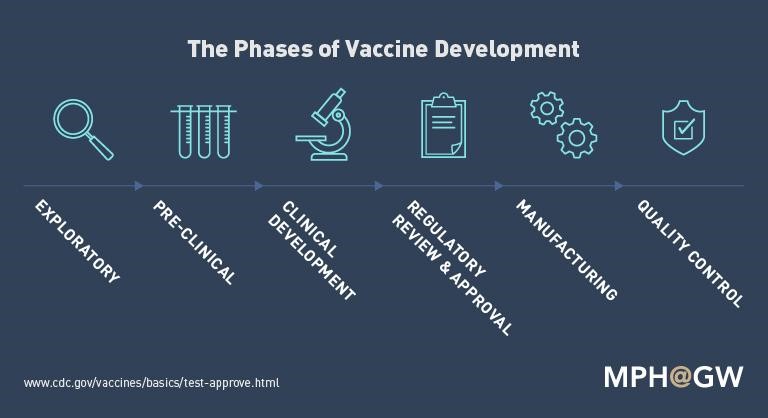
- Exploratory stage – Basic laboratory research takes place during this stage.
- Pre-clinical stage – Officials begin testing with animals and tissue-culture or cell-culture systems. Researchers then get an idea of the immune response they can expect the potential vaccine to provoke in humans.
- Clinical development – Clinical development is a 4-phase process.
Phase I – a phase with small groups of people receiving the trial vaccine.
Phase II – an expanded trial group with vaccination recipients who display similar characteristics to potential vaccine users.
Phase III – a considerably larger phase with thousands of vaccination recipients. Testing is focused on efficacy and safety.
Phase IV – a phase that many companies choose to use to conduct extra, ongoing studies after the vaccine has already been approved and licensed.
- Regulatory review and approval – this step can only come after detailed applications and rigorous demonstration of good manufacturing practices. Regulatory review and approval processes then allow the manufacturer to receive FDA licensing.
- Manufacturing – During this part of the process, vaccines are made.
- Quality control – Using information from the public and healthcare providers, quality control continues through different agencies/methods long after a vaccine is licensed, manufactured, and distributed.
What coronavirus vaccines are currently in development
Until today, 8 vaccines are currently being tested in human clinical trials. While some trials are in phase II, it could still be many months before we know whether any of these vaccines will protect people against COVID-19. (Bloomberg, 2020)
| Institutions | Country | Type of vaccine | Status |
| CanSino Biological and the Beijing Institute of Biotechnology | China | Non-replicating viral vector | Phases I and II |
| Moderna and the National Institute of Allergy and Infectious Diseases | U.S. | RNA | Phases I and II |
| Inovio Pharmaceuticals | U.S. | DNA | Phase I |
| Beijing Institute of Biological Products and Sinopharm | China | Inactivated | Phases I and II |
| Wuhan Institute of Biological Products and Sinopharm | China | Inactivated | Phases I and II |
| Sinovac | China | Inactivated | Phases I and II |
| University of Oxford | UK | Non-replicating viral vector | Phases I and II |
| BioNTech/Fosun Pharma/Pfizer | U.S. and Europe | RNA | Phases I and II |
When Will We Have a Vaccine?
Researchers say that even if initial trials of vaccines go well, it could be a year to a year and a half before a vaccine is ready for use by the general public. Since it typically takes 10 to 15 years to develop a vaccine, this would be an incredibly accelerated timeline.
Once the vaccine is approved, it will still need to be mass-produced. Early on, supplies would probably be limited and go to higher priority groups such as healthcare workers, kids, and the elderly.
Does the flu vaccine work against COVID-19?
There are vaccines for many different types of viruses and bacteria. However, a vaccine that works against one type of virus, such as influenza A (which can cause the seasonal flu), won’t work against another type, like SARS-CoV-2 (the coronavirus that causes COVID-19). The flu vaccine is designed to target strains of influenza, and won’t protect against the coronavirus, which is an entirely different virus. (GoodRx, 2020)
Meanwhile, the latest update says – researchers at Oxford University, where one of the leading efforts to develop a vaccine is underway, also said a vaccine might be available as soon as September.
The university further claimed in a press release that under the best-case scenario, it could have an efficacy result from a phase-three trial by this fall “to show that the vaccine protects against the virus, alongside the ability to manufacture large amounts of the vaccine.”
In the U.S., the Trump administration is organizing a Manhattan Project-style effort to drastically cut the time needed to develop a coronavirus vaccine, with the goal of making 300 million doses of vaccine available by January.
The effort by the U.S. government is so intense that it is building production lines before it has a vaccine ready to produce.
Work Cited
“In Coronavirus Vaccine Hunt, a Race to Be First.” Voice of America, www.voanews.com/covid-19-pandemic/coronavirus-vaccine-hunt-race-be-first
Molteni, Megan. “Front-Runners Emerge in the Race for a Covid-19 Vaccine.” Wired, Conde Nast, www.wired.com/story/frontrunners-emerge-in-the-race-for-a-covid-19-vaccine/
Bloomberg.com, ‘’The Race to Develop a Coronavirus Vaccine: What you need to know”, www.bloomberg.com/news/storythreads/2020-05-08/the-race-to-develop-a-coronavirus-vaccine
Wagener, Dan. “Live Updates: The Race for a Coronavirus (COVID-19) Vaccine – GoodRx.” The GoodRx Prescription Savings Blog, 12 May 2020, www.goodrx.com/blog/coronavirus-covid-19-vaccine-availability-live-updates/
“Vaccine Testing and Approval Process.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 1 May 2014, www.cdc.gov/vaccines/basics/test-approve.html